Plastic to Oil Distillation

Fractional distillation differs from distillation only in that it separates a mixture into a number of different parts, called fractions. A tall column is fitted above the mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points condense at the bottom and substances with low boiling points condense at the top. Like distillation, fractional distillation works because the different substances in the mixture have different boiling points.
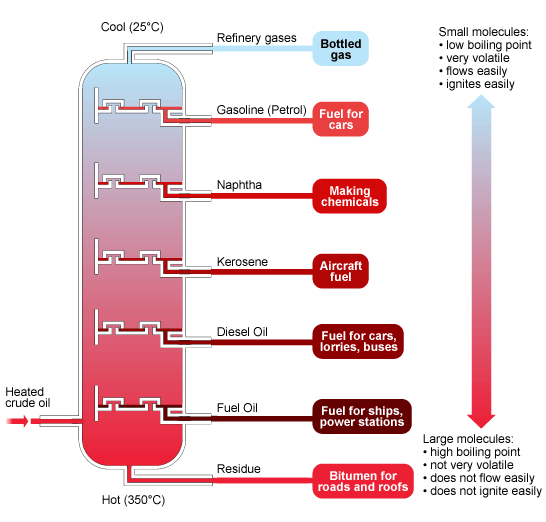
The diagram above summarizes the main fractions from crude oil and their uses, and the trends in properties. Note that the gases condense at the top of the column, the liquids in the middle and the solids stay at the bottom.
The main fractions include refinery gases, gasoline (petrol), naphtha, kerosene, diesel oil, fuel oil, and a residue that contains bitumen. These fractions are mainly used as fuels, although they do have other uses too.
Hydrocarbons with small molecules make better fuels than hydrocarbons with large molecules because they are volatile, flow easily and are easily ignited.
Pyrolytic Fractional Distillation Waste Polymers Scrap Plastics
